


#As element configuration full#
This is called Hund's Rule: "Half fill before you Full fill" and again this rule was established based on energy calculations that indicated that this was the way atoms actually distributed their electrons into the orbitals. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before filling them with both electrons. The boxes are used to represent the orbitals and to show the electrons placed in them. There are lots of quizzes on electron configurations you can practice with located here Orbital DiagramsĪnother way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": In these columns, the 4s and 3d Practice, Practice, Practice In the d block, specifically the groups containing Chromium and Copper, there is an exception in how they are filled. But based on the electron configurations that are generated, these exceptions are easy to understand. You just have to finish the configuration from where the noble gas leaves it:Īs with every other topic we have covered to date there are exceptions to the order of fill as well. In these cases, you can use the previous noble gas to abbreviate the configuration as shown below. When writing some of the lower table configurations the total configuration can be fairly long. One other note on writing electron configurations: A short cut.
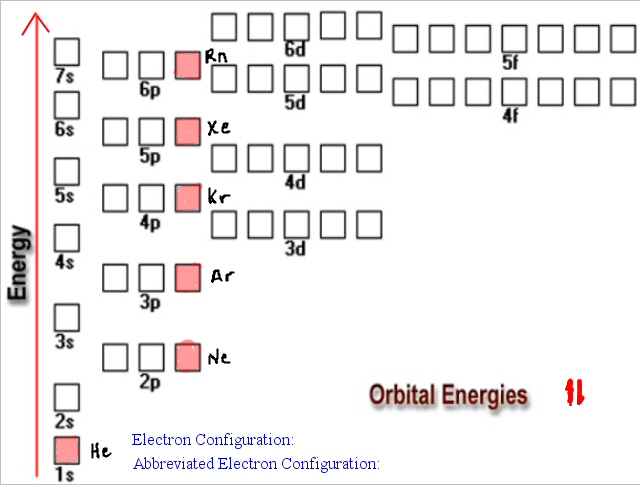
When we make a 3+ ion for Iron, we need to take the electrons from the outermost shell first so that would be the 4s shell NOT the 3d shell: Fe 3+ 1s 22s 22p 63s 23p 63d 5 Iron has 26 electrons so its normal electron configuration would be: Fe 1s 22s 22p 63s 23p 64s 23d 6 Note that this is not always the same way they were added. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. The electron configurations for Cations are also made based on the number of electrons but there is a slight difference in the way they are configured. We talked about the fact that ions form because they can become more stable with the gain or loss of electrons to become like the noble gases and now you can actually see how they become the same. With 10 electrons you should note that oxygen's electron configuration is now exactly the same as Neon's. This would add 2 electrons to its normal configuration making the new configuration: O 2- 1s 22s 22p 6. If you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued.įor example, we know that Oxygen always forms 2- ions when it makes an ion. Special CasesĬonfigurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place. So Oxygen's electron configuration would be O 1s 22s 22p 4. Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. Looking at the periodic table, you can see that Oxygen has 8 electrons. The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. Just like the quantum numbers themselves this order was determined by calculation and is summarized by the following chart: This is referred to as the Aufbau principle. The order in which electrons are placed into the orbitals is based on the order of their energy. BUT what we haven't discussed is how these orbitals get filled.the order of fill. So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. Here is a summary of the types of orbitals and how many electrons each can contain: What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons.

Electron ConfigurationĮlectron configurations are the summary of where the electrons are around a nucleus. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. The content that follows is the substance of General Chemistry Lecture 26. Electron Configurations Electron Configurations


 0 kommentar(er)
0 kommentar(er)
